Macu4
Challenges
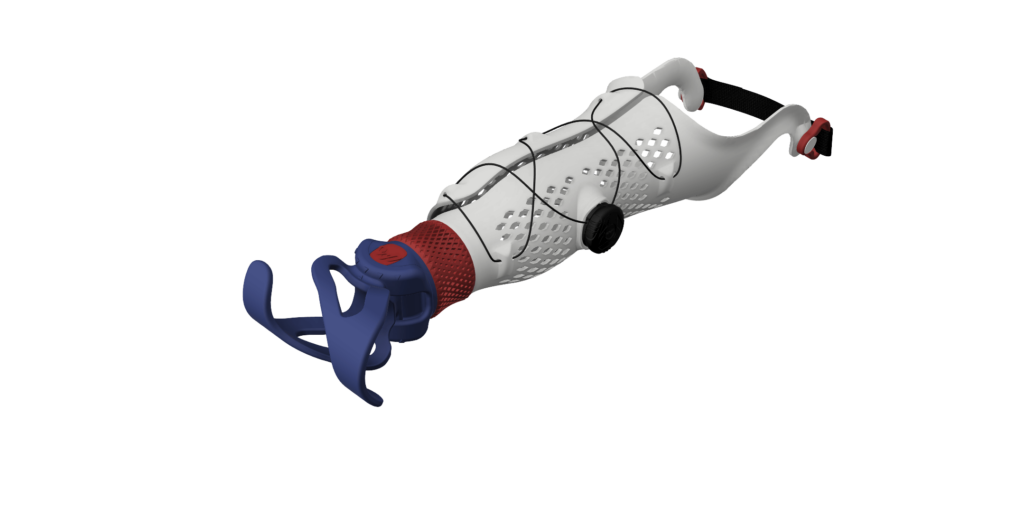
Macu4 specialise in the production of forearm prostheses for outdoor activities; customers upload their measurements and order bespoke, 3D printed parts for a variety of different functions. Subsequently, each unique customer part needs to display compliance with the Medical Device Regulation (MDR) as well as a Global Trade Item Number (GTIN) for quality assurance purposes. Additionally, Macu4 wanted to be able to configure customer orders and automatically generate reports, packing lists and other order-specific information to streamline their production process.
Solution
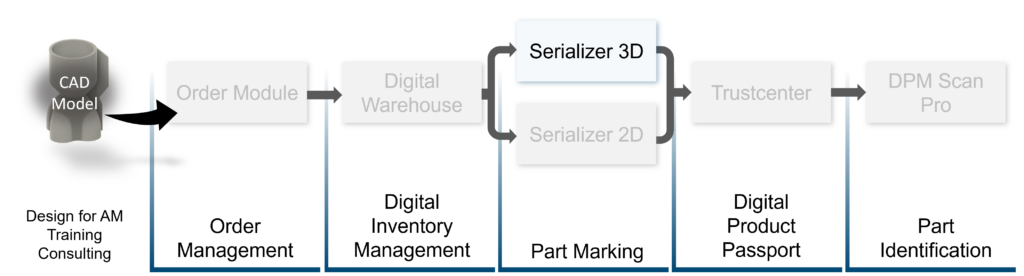
Through the application of our Serializer, Macu4 was able to apply direct part markings to their components (including GTINs), proving product authenticity and supporting compliance with the European Medical Device Regulations. This compliance is crucial as it guarantees the safety and effectiveness of the devices, thereby fostering trust among end-users and regulatory bodies.
In addition, our solutions support Macu4 with digital inventory management, simplifying and automating the ordering process; as well as developing a UI to allow Macu4 to configure customer orders, every order is accompanied by automatically-generated report containing comprehensive information about the parts in the order. Furthermore, our system generates two versions of the inventory following every order – the original parts and marked part files – which adds additional security should an issue arise during the manufacturing phase.
Overall, through the implementation of our Additive Marking Suite, Macu4 achieved greater production efficiencies, enhancing customer satisfaction by delivering products that meet the highest standards of quality and compliance. The one-time set up of the order configurator and the automatic generation of reports is an easily transferable workflow, which can bring benefits to companies across a wide range of industries.

